ctx clinical trial
The RESTORE study is a Phase 3 clinical trial looking at an investigational medication called chenodeoxycholic acid also called Chenodal or CDCA. KANAGAWA Japan June 7 2022 PRNewswire -- Chordia Therapeutics Inc.
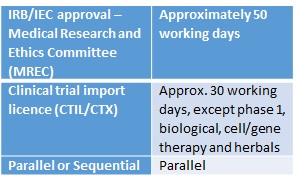
Regulatory Timelines In The Asia Pacific
This is an open-label multicenter Phase 1 study evaluating the safety and efficacy of CTX110 in subjects with relapsed or refractory B-cell malignancies.
. Chenodal is not indicated for the treatment of CTX but has received a medical. Participate and supplement your income. Ad PPMI is changing how patients and scientists think about brain disease.
Transition to a Simpler More Agile Clinical Trial Experience. Since the last publication of Guideline for the application of Clinical Trial Import Licence CTIL and Clinical Trial Exemption CTX 5th Edition in 2009 we have witnessed robust growth in. Clinical trials of medicines and biologicals regulated under the CTN or CTA schemes are subject to the TGAs Good Clinical Practice GCP Inspection Program.
ClinicalTrialsgov is a database of privately and publicly funded clinical studies conducted around the world. Now it needs you. CTX is a rare progressive disorder that can affect the brain spinal cord tendons eyes and arteries.
Find local clinical trial opportunities. Clinical Trial Exemption CTX scheme renamed as Clinical Trial Approval CTA scheme 6 November 2020 The Therapeutic Goods Administration TGA has changed the. Ad See Why Medable Was Just Named The 1 Decentralized Clinical Trial Platform By Everest.
The Most Utilized Decentralized Clinical Trial Platform Globally. Vertex-CRISPRs CTX001 shows positive outcomes in trial patients Vertex Pharmaceuticals and CRISPR Therapeutics have reported positive interim results from two. PPMI is changing how patients scientists think about brain disease.
Interventional Clinical Trial Estimated Enrollment. Local and national research studies. A Safety and Efficacy Study Evaluating CTX130 in Subjects With Relapsed or Refractory Renal Cell Carcinoma COBALT-RCC The safety and scientific validity of this study.
Ad Combat Data-related Challenges Remove Data Bottlenecks in Your Clinical Trials. CRISPR Therapeutics and Vertex have launched two Phase 3 trials to assess the safety and effectiveness of CTX001 an experimental gene-editing cell therapy one in children. None Open Label Primary.
See If You Qualify For Any In Your Area. Clinical Trials Patient Registry - Cerebrotendinous Xanthomatosis CTX Clinical Trials Patient Registry Clinical Research The investigational therapies explored in clinical trials are key to. CTX Clinical Trial Exemption An approval process.
Clinical Trials Xpress CTX is an initiative of the University of Texas System established to provide an efficient and scalable centralized operating model for conducting multi-site clinical. The Therapeutic Goods Administration TGA directly reviews the planned clinical trial and must give their approval for. Ad Access To New Treatments In Clinical Trials.
Chordia a biotech company engaged in the research and development of novel therapies for. Ad PPMI is changing how patients and scientists think about brain disease. Now it needs you.
See listed clinical studies related to the coronavirus disease COVID-19 ClinicalTrialsgov is a resource provided by the. Explore 424674 research studies in all 50 states and in 221 countries. Ad Clinical studies advance scientific knowledge.
PPMI is changing how patients scientists think about brain disease. We are inviting people with. This is a Phase 1 open-label first-in-human study of CTX-471 monotherapy in patients with metastatic or locally advanced malignancies that have progressed while receiving.
Explore 406391 research studies in all 50 states and in 220 countries. Eye clinical trials xplained Eye CTX The Eye CTX storyboard was created by doctors and the Roche Ophthalmology Patient Council members representing those living with vision.

Clinical Trials Medical Device Trials Genesis Research Services

Introduction To Changes To The Tga S Clinical Trial Notification Ctn

Regulatory Requirements For Clinical Trials Australia Vs The Us
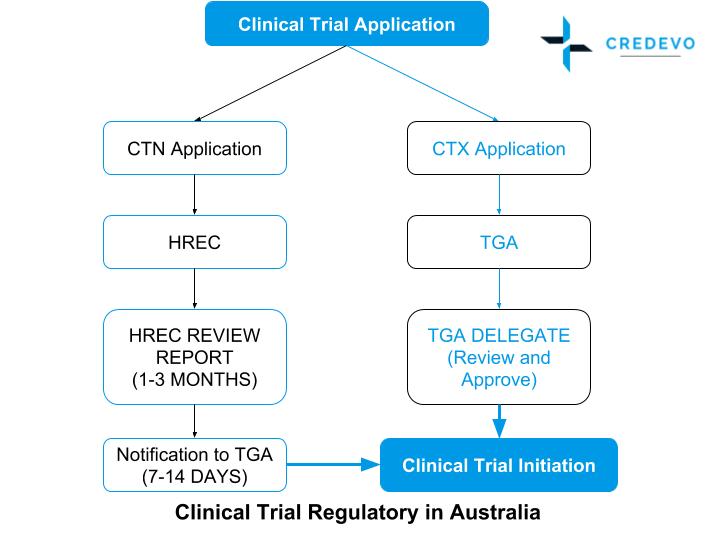
How To Get Started With Your Clinical Trials In Australia

Efficacy Clinical Trial Data Crysvita Burosumab Twza For Tio
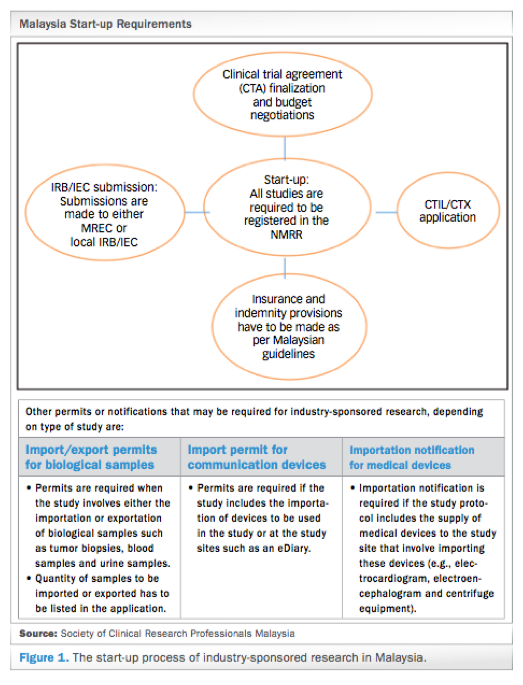
Malaysia S Clinical Research Ecosystem

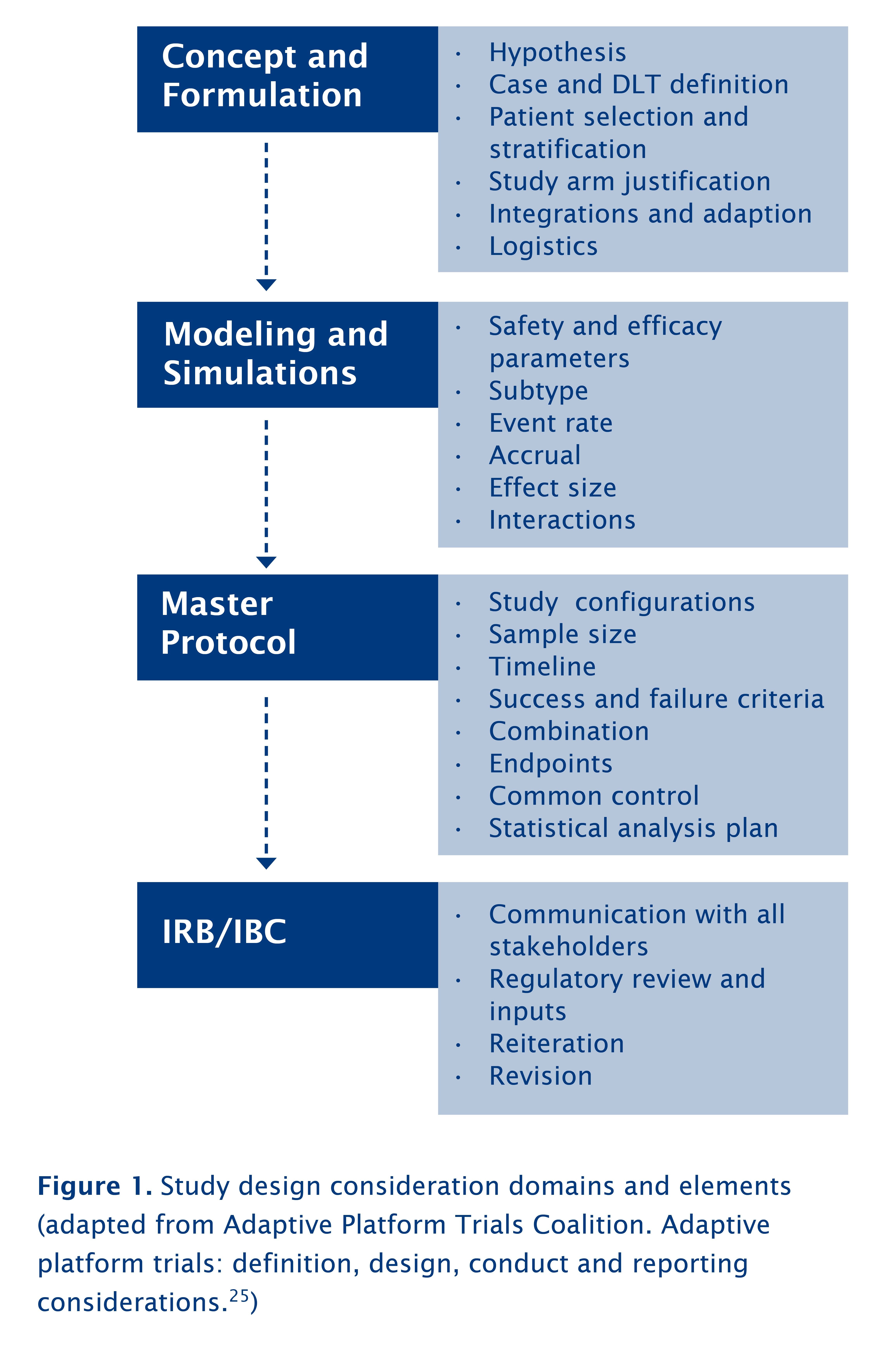
Initial Design Considerations Of Trials Of Immuno Oncology Domains And Elements

Introduction To Changes To The Tga S Clinical Trial Notification Ctn

Clinical Trials Medical Device Trials Genesis Research Services

Regulatory Timelines In The Asia Pacific George Clinical

Comparison Of The Eu Cta And The Us Ind Application Procedures For Download Scientific Diagram
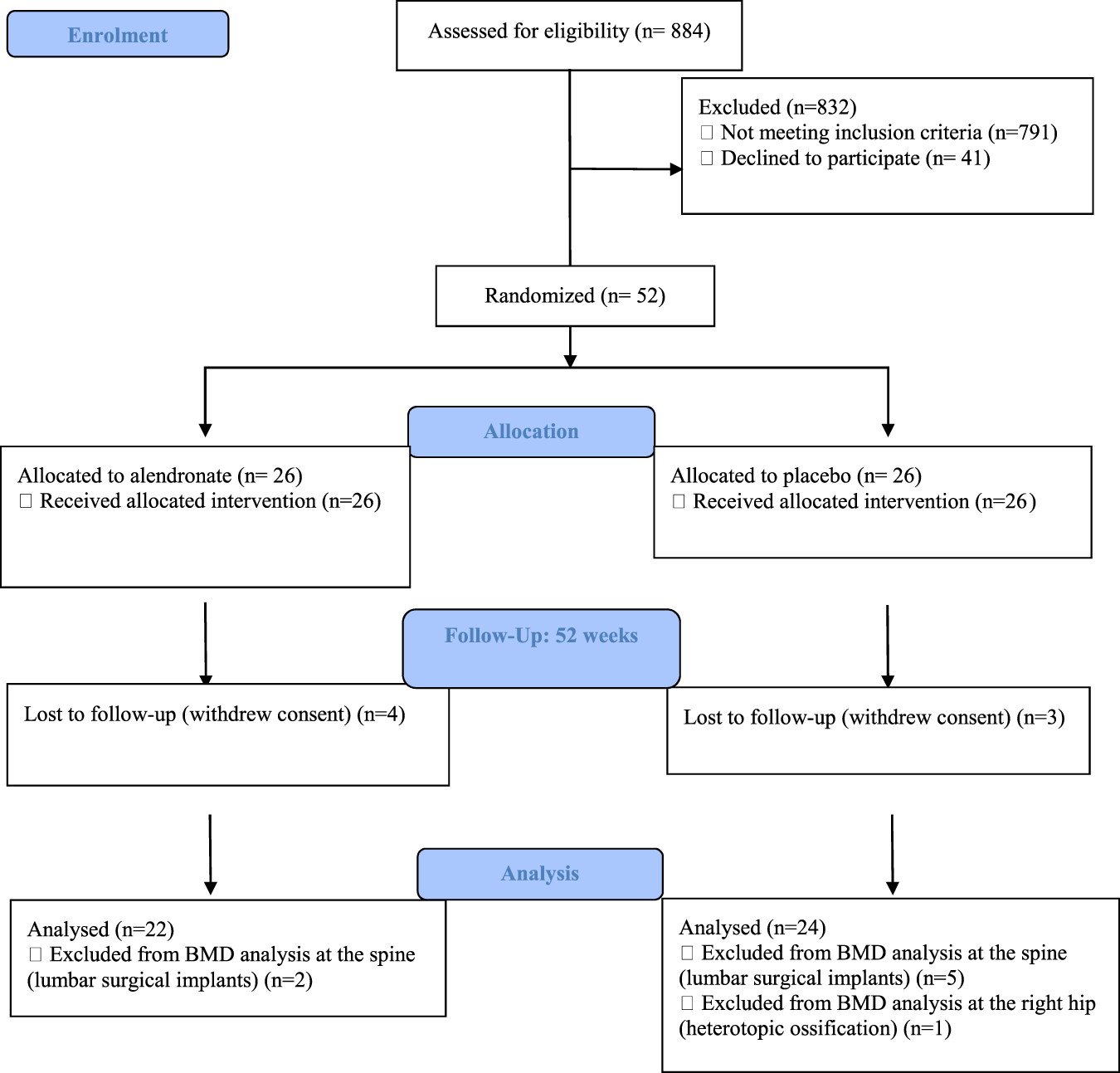
Preventive Treatment With Alendronate Of Loss Of Bone Mineral Density In Acute Traumatic Spinal Cord Injury Randomized Controlled Clinical Trial Spinal Cord

Introduction To Changes To The Tga S Clinical Trial Notification Ctn

Active Trials Head And Neck Cancer Alliance

Study Design And Rationale For The Pace Lung Trial A Multicenter Single Arm Phase Ii Clinical Trial Evaluating The Efficacy Of Additional Chemotherapy For Patients With Egfrm Nsclc With The Continued Presence Of Plasma
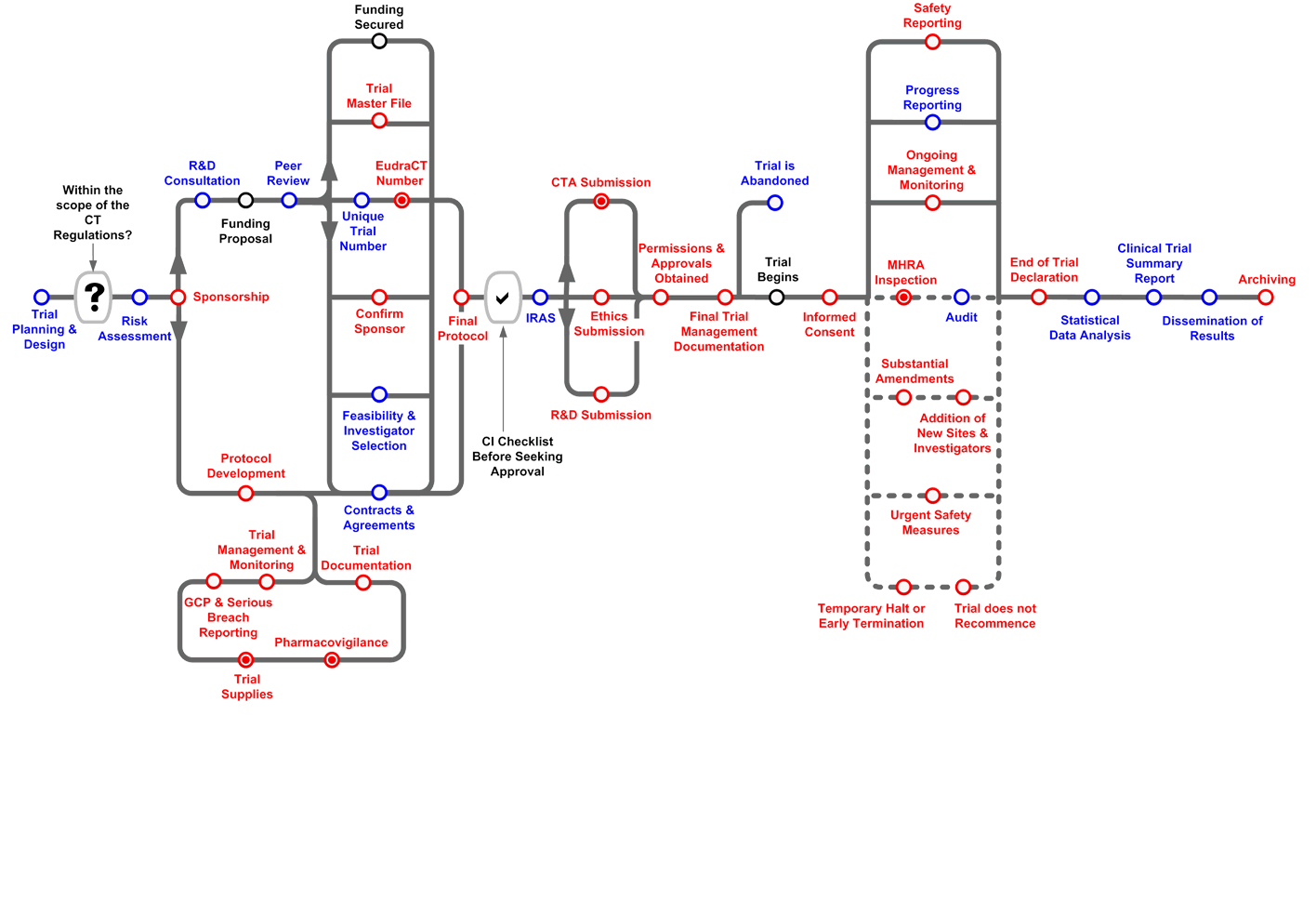



Comments
Post a Comment